Constipation
Disclaimer
These guidelines have been produced to guide clinical decision making for the medical, nursing and allied health staff of Perth Children’s Hospital. They are not strict protocols, and they do not replace the judgement of a senior clinician. Clinical common-sense should be applied at all times. These clinical guidelines should never be relied on as a substitute for proper assessment with respect to the particular circumstances of each case and the needs of each patient. Clinicians should also consider the local skill level available and their local area policies before following any guideline.
Read the full CAHS Emergency Department disclaimer
|
Aim
To guide Emergency Department (ED) staff with the assessment and management of constipation.
Definition
Constipation is a symptom not a disease. Constipation refers to infrequent bowel movements or hard to pass faeces.
Background
- Constipation in children is most commonly due to a functional cause (95%)1.
- Although rare, some causes of constipation are potentially life threatening.
Constipation can present as:
- History of infrequent stools (< 3 stools per week).
- Large and/or hard stool associated with painful defaecation.
- Intermittent abdominal pain.
- Incomplete evacuation of rectal content.
- Involuntary soiling.
- Inability to pass stool.
Normal stool pattern
- A breast fed baby may pass a stool after every feed ranging to a stool only every 7-10 days2.
- A bottle fed baby and older child will usually pass a stool every 1-2 days2.
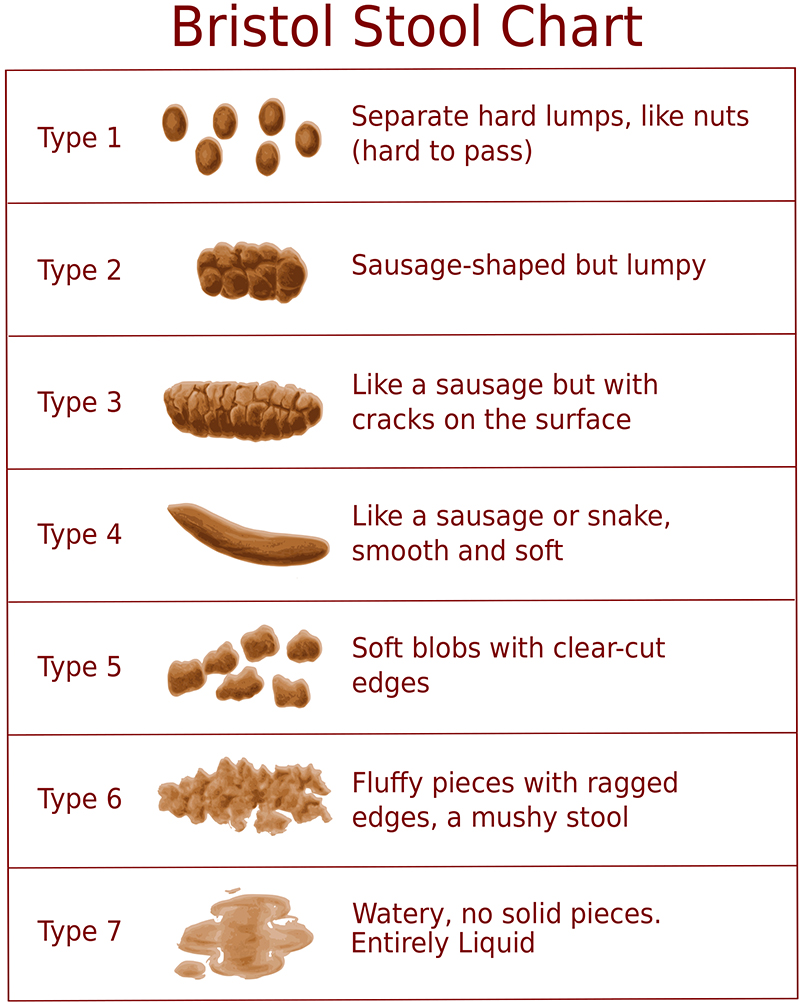
- See Bristol Stool Chart below3:
- Types 1 and 2 indicate constipation.
- Types 3 and 4 indicate the ideal stool.
- Types 5-7 indicate potential diarrhoea.
History
- Delayed passage of meconium for more than 48 hours.
- Constipation present from birth or early infancy.
- Failure to thrive, significant weight loss.
- Abdominal distention, bilious vomit or ileus.
- Child is systemically unwell, fever, vomiting.
- Fatigue, polyuria, polydipsia.
- Urinary incontinence.
- Extraintestinal symptoms.
Examination
- Lower spine abnormalities.
- Decreased lower limb tone, reflex or strength.
- Unexplained abdominal or pelvic mass.
- Patulous anus, anal prolapse, anteriorly placed anus.
- Blood in stool not attributed to anal fissure.
- Representation, or failed standard treatment.
Assessment
History
The evaluation of the child presumed to have constipation should begin with a thorough history and physical examination with special attention to red flags.
- Age of onset of constipation, duration, frequency of episodes, time of first meconium after birth.
- Stool frequency, consistency and size (see Bristol Stool Chart).
- Defaecation – painful or straining.
- Any blood on stool or toilet paper.
- Precipitating factors - diet, environment, psychosocial history, etc.
- Any stool incontinence – encopresis, soiling, overflow, diarrhoea.
- Associated abdominal pain or vomiting.
- Child’s general health and associated recent illness, failing to thrive, developmental history, etc.
- Treatment / medication used and response.
Examination
- Digital rectal examination is not routinely performed by Emergency Department Clinicians
Investigations
- An abdominal X-Ray is rarely indicated, although exceptions may include:
- Child with faecal soiling who does not have a faecal mass palpable.
- Child who is markedly obese.
Management
- A general rule of thumb is that treatment is required to maintain soft stools for as long as the patient has been constipated (e.g. if constipated for one year, it is likely they will require treatment for a year).
Risks in management
- Electrolyte imbalance can occur as the use of laxative solutions provides no net fluid input and there is a risk of dehydration and/or electrolyte imbalance if adequate fluid intake is not maintained during treatment.4
- Impaired absorption of medications can result if taken within an hour of commencing colonic lavage therefore the patient’s usual medication regimen should be considered and may need to be adjusted prior to commencing treatment.
Initial management
- Disimpaction is necessary before initiation of maintenance therapy.
Disimpaction
- The oral approach to disimpaction is not invasive and gives a sense of power to the child, but adherence to the treatment regimen may be a problem.
- The rectal approach to disimpaction is faster but is invasive.
- The oral and rectal approach may both be required.
- The choice of treatment is best determined after discussing the options with the Emergency Department Senior Doctor and the family.
- Ensure the patient is provided with a Constipation Management Plan, completed using the information below.
Rectal disimpaction
Glycerol5
- < 4 weeks old refer to Glycerol (Glycerine) – Neonatal Monographs
- 4 month – 1 year, rectal 700 mg once daily when required.
- 1–12 years, rectal 1.4 g once daily when required.
- 12–18 years, rectal 2.8 g once daily when required.
- Ensure the child retains the suppository for 15-30 minutes.
- Enemas are usually reserved for children with severe rectal pain or distress due to faecal impaction.
- Discuss use with Emergency Department Senior Doctor.
|
|
Phosphate enemas (Fleet®)
Refer to Phosphate Monograpgh (Medication Management Manual) (internal WA Health only)
- Contraindicated in <2 years old, gastrointestinal obstruction or perforation, congenital megacolon i.e. Hirschsprung’s disease, or renal failure.6
Onset of action is up to 30 minutes after rectal administration. Avoid if possible due to increased risk of renal damage (including acute renal failure), dehydration and electrolyte disturbance (deaths have occurred).7
- Rectal dose: Using Fleet® ready-to-use enema:8
- 3 years – <7 years: 40-60 mL (approximately ⅓ - ½) once daily.
- 7 years – <12 years: 60-90 mL (approximately ½ - ¾) once daily.
- 12 years – 17 years: 90-118 mL (approximately ¾- whole bottle) once daily.
- Administration6
- unscrew cap, remove and discard the excess volume of liquid then replace cap and administer.
- it is not necessary to empty the bottle completely, as it contains more liquid than is needed.
|
|
Microlax® or Microlette® micro-enema (contains sodium citrate, sodium lauryl sulfoacetate and sorbitol) 7
- Suitable for children > 4 weeks old
- Dose: 5 mL rectally as a single dose
- Insert only half the nozzle length for children < 3 years
|
Oral disimpaction
- Macrogol 3350 with electrolytes is the recommended laxative (e.g. Movicol®, Macrovic® and many other brands).9
- Adult Macrogol 3350 with electrolytes is equivalent to two Junior preparations (i.e. 1 regular sachet = 2 junior sachets). Various brands are available, consult with pharmacy if advice is required.
- Palatability is improved by adding flavour e.g. cordial9
- Disimpaction usually takes 3-5 days but may take longer. If taking longer than 7 days, continue with day 7 dose until disimpaction occurs.
- Add a stimulant laxative after 2 weeks if disimpaction has not been achieved.
- Stop once disimpaction occurs and then commence maintenance dose. A child may require treatment to maintain soft stools for many weeks or months. This is not a quick fix. If treatment is stopped and constipation reoccurs, start the medication again and review in 3 months.
| 4 weeks - 12 months |
¼ - ½ sachet |
¼ - ½ sachet |
¼ - ½ sachet |
¼ - ½ sachet |
¼ - ½ sachet |
¼ - ½ sachet |
¼ - ½ sachet |
Continue with day 7 dose until disimpaction occurs
|
| 1-6 years |
1 sachets |
2 sachets |
2 sachets |
3 sachets |
3 sachets |
4 sachets |
4 sachets |
| 6-12 years |
2 sachets |
3 sachets |
4 sachets |
5 sachets |
6 sachets |
6 sachets |
6 sachets |
| 12-18 years |
8 sachets |
8 sachets |
8 sachets
|
8 sachets |
8 sachets |
8 sachets |
8 sachets |
Cardiovascular disease risk due to salt content: do not exceed >2 sachets/hour
Alternatively, if macrogol 3350 with electrolytes is not tolerated, use macrogol 3350 without electrolytes (OsmoLax®).
Number of OsmoLax® small scoops (8.5g)
| 2-6 years |
2 scoops |
3 scoops |
3 scoops |
4 scoops |
5 scoops |
6 scoops |
6 scoops |
6 scoops |
| 6-12 years |
3 scoops |
4 scoops |
6 scoops |
8 scoops |
9 scoops |
9 scoops |
9 scoops |
9 scoops |
When to consider admission for oral or nasogastric colonic lavage if:
- Disimpaction is not achieved with the rectal or oral medication.
- Large amounts of faeces are palpable through the abdominal wall.
- ColonLYTELY® (macrogol 3350 with sodium sulfate and electrolytes) is recommended for children > 1 year10 to be given at a dosage of 60-100 mL/kg/day (up to a maximum of 3 L/day)11 and can be given orally or via nasogastric tube.
- The solution should be consumed or administered via nasogastric tube over the shortest duration that the child can tolerate but should take no longer than 10 hours to complete. (Note. The maximum rate that can be administered by the Kangaroo ePumpTM enteral feeding pump is 300 mL/hr).
- Aim to complete the treatment prior to the patient’s bedtime to enable rest overnight.
- Refer to Colonic Lavage for Management of Faecal Impaction and Chronic Constipation (internal WA Health only – Clinical Practice Manual).
- Alternatively, PicoPrep® (sodium picosulfate, magnesium oxide, citric acid) may be used.4 Refer to the Magnesium Monograph (internal WA Health only - Medication Management Manual) for information on dose and administration (as per bowel preparation prior to colonoscopy).
Further management
- Behavioural modification should include regular scheduled toileting for approximately five minutes after each meal.
Maintenance treatment
- First Line Laxative treatment:
- Children < 2 years, stool softener and/or osmotic laxative.
- Children > 2 years, osmotic laxative.
- Macrogol 3350 with electrolytes is the recommended laxative (e.g. Movicol®, Macrovic® and many other brands).
- Long term treatment needs to be under the supervision of the child’s local doctor.
Medication
Osmotic agents
Macrogol 3350 with electrolytes9 e.g. Movicol®
Macrogol 3350 with electrolytes9 (Adult) Doses:
- 4 weeks to 1 year: oral, ¼ - ½ sachet daily.
- > 1 - 6 years: oral, initially ½ sachet per day (maximum 2 sachets daily)
- > 6 - 12 years: oral, initially 1 sachet per day (maximum 2 sachets daily)
- > 12 years: oral, initially 1 sachet per day (maximum 3 sachets daily)
*Note: Use double the dose if using Junior preparations (i.e. 1 regular sachet = 2 junior sachets. If unsure, please consult with your pharmacist.)
Macrogol 3350 without electrolytes9 e.g. OsmoLax®
Measure powder using double-ended 8.5 g and 17 g scoop provided.
- 2 – 6 years: oral, initially 8.5 g once daily; maximum 17 g daily.
- > 6 – 12 years: oral, initially 17 g once daily; maximum 25.5 g daily.
- >12 years: oral, initially 17 g once daily; maximum 34 g daily.
Lactulose or Sorbitol12,13
- 4 weeks to 1 year: oral, 2.5 mL BD.
- > 1 year - 5 years: oral, 2.5 – 10 mL BD.
- > 5 years: oral, 5 – 20 mL BD.
- Up to 1.5 mL/kg BD. Daily maximum is 60 mL.
Stool softener
Poloxamer (Coloxyl Infant Drops®)14
- < 6 months: oral, 0.3 mL TDS.
- 6 - 18 months: oral, 0.5 mL TDS.
- 18 - 36 months: oral, 0.8 mL TDS.
Paraffin emulsion 50% (Parachoc®)15
- 1 - 6 years: oral 10-15 mL once daily.
- > 6 - 12 years: oral 20 mL once daily.
- > 12 years: oral 40 mL once daily.
References
- Loening-Baucke V, Swidsinski A. Constipation is the Most Frequent Cause of Chronic Abdominal Pain in Children. The Open Pediatric Medicine Journal. 2008, 2, 16 - 20
- Steer, Emond & Sandhu. The variation in stool patterns from 1 to 42 months: a population-based observational study. Archives of Disease in Childhood 2009;94:231-233
- Lewis, SJ.; Heaton, KW. (September 1997). "Stool form scale as a useful guide to intestinal transit time". Scand J Gastroenterol. 32 (9): 920–4. doi:10.3109/00365529709011203. PMID 9299672.
- Royal Children's Hospital, Melbourne, Australia, Clinical Practice Guideline on Constipation, [Internet, last updated October 2017; cited 31 August 2020], Available from: https://www.rch.org.au/clinicalguide/guideline_index/Constipation/
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Glycerol [Modified: January 2022 Cited: 2 February 2022] Available from: Glycerol - AMH Children's Dosing Companion (health.wa.gov.au)
- Clinical Pharmacology. Last Updated:31 Dec 2018. Cited 7 September 2022. Available From: Sodium Phosphate Monobasic Monohydrate; Sodium Phosphate Dibasic Anhydrous Contraindications/Precautions - Clinical Pharmacology (health.wa.gov.au)
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Saline Laxatives [Modified: January 2022 Cited: 2 February 2022] Available from: Saline laxatives - AMH Children's Dosing Companion (health.wa.gov.au)
- Paediatric Formulary Committee. BNF for Children [Internet]. London: BMJ Group, Pharmaceutical Press, and RCPCH Publications. Sodium acid phosphate with sodium phosphate [Last update 2021 Oct 18 Cited 2022 Sept 9]. Available from: https://www-medicinescomplete-com.pklibresources.health.wa.gov.au/#/content/bnfc/_362863307?hspl=fleet
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Macrogol Laxatives [Modified: January 2022 Cited: 2 February 2022] Available from: Macrogol laxatives - AMH Children's Dosing Companion (health.wa.gov.au)
- Polyethylene glycol and electrolyte solution Drug information, Dosing: Paediatric. Lexicomp Inc. UpToDate. Cited 7 September 2022. Available from: Polyethylene glycol and electrolyte solution: Drug information - UpToDate (health.wa.gov.au)
- South Australian Child and adolescent health service, Australia, Paediatric clinical practice guidelines constipation in children. [Internet, last updated May 2020; cited 31 August 2020], Available from https://www.sahealth.sa.gov.au/wps/wcm/connect/f67aeb004329b2228184ed8bf287c74e/Constipation_in_+Children_Paed_v4.1.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-f67aeb004329b2228184ed8bf287c74e-n8V9oFA
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Lactulose [Modified: January 2022 Cited: 2 February 2022] Available from: Lactulose - AMH Children's Dosing Companion (health.wa.gov.au)
- Sorbitol Drug information, Dosing:Paediatric. Lexicomp Inc. UpToDate. Cited 7 September 2022. Available from: Sorbitol: Drug information - UpToDate (health.wa.gov.au)
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Poloxamer [Modified: January 2022 Cited: 2 February 2022] Available from: Poloxamer - AMH Children's Dosing Companion (health.wa.gov.au)
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Paraffin Emulsion [Modified: January 2022 Cited: 2 February 2022] Available from: Paraffin emulsion - AMH Children's Dosing Companion (health.wa.gov.au)
| Endorsed by: |
CAHS Drugs and Therapuetics Committee |
Date: |
Dec 2024 |
This document can be made available in alternative formats on request for a person with a disability.