Median nerve block
Disclaimer
These guidelines have been produced to guide clinical decision making for the medical, nursing and allied health staff of Perth Children’s Hospital. They are not strict protocols, and they do not replace the judgement of a senior clinician. Clinical common-sense should be applied at all times. These clinical guidelines should never be relied on as a substitute for proper assessment with respect to the particular circumstances of each case and the needs of each patient. Clinicians should also consider the local skill level available and their local area policies before following any guideline.
Read the full CAHS clinical disclaimer.
|
Aim
To guide PCH Emergency Department (ED) staff in the use of median nerve block.
Background
A median nerve block uses local anaesthetic to block the nerve at the wrist, allowing procedures on the radial side of the palm, palmar surface and tip of the thumb, index and middle finger and (variably) the ring finger.
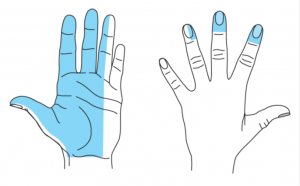
Pre-procedure
Indications1
- Suitable for use in injuries requiring procedures on the radial side of palm, palmar surface and tip of the thumb, index, middle and ring fingers where treatment of duration less than 30-45 minutes such as:
- finger or hand lacerations requiring suturing
- removal of foreign body from palm or medial fingertips
- part of a hand block (with radial and ulnar nerve blocks)
- Not suitable for joint or fracture manipulation without doing a radial nerve block as well
Requirements
- The patient needs to be able to cooperate with injections and hold still for the procedure intended.
- Formal consent not required but procedure should be clearly documented.
- A clear explanation of the procedure needs to be provided to the patient
- Risks need to be explained:
- Pain as the injection is made.
- Nerve block might not work.
- Bruising and bleeding at the site of injection.
- Ensure the patient is in the appropriate treatment area.
Preparation
Equipment and dosing
- Sterile gloves and dressing pack with antiseptic (chlorhexidine 2% or similar).
- 5mL syringe filled with lidocaine (lignocaine) 1% (50mg/5mL) or lidocaine (lignocaine) 2% (100mg/5mL) without adrenaline (epinephrine)
- Maximum dose of lidocaine (lignocaine) is 3 mg/kg without adrenaline (epinephrine) (not to exceed adult maximum of 200mg).4,5
OR
- 5mL syringe filled with lidocaine (lignocaine) 1% with adrenaline (epinephrine) 1:100,000 mL.
- Maximum dose of lidocaine (lignocaine) is 7 mg/kg (not to exceed adult maximum of 500mg) with adrenaline (epinephrine).4,6
- Warm lidocaine (lignocaine) to body temperature (i.e. in your hand) to reduce discomfort.
- 25 gauge needle (orange) for the injection
- Consider topical local anaesthetic e.g. lidocaine (lignocaine) with prilocaine (EMLA®) application over injection site.
- Consider Nitrous Oxide for sedation whilst injections are occurring.
Procedure
Positioning and technique
- Position patient with palm held upwards and slightly flexed
- Drape wrist appropriately and prepare with aseptic technique
Ultrasound guided technique
- Ultrasound guidance increases chance of a successful block and may be more appropriate particularly in younger children in whom identifying anatomical landmarks is difficult7
- Use a high-frequency linear transducer (>10 MHz) and a sterile probe cover8
- Place the probe over the volar wrist in the transverse plane between radial and ulnar arteries and move proximally until the median nerve can be visualised as a hyperechoic oval structure between flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP)7,9
- Advance 25-27 gauge needle using an in-plane approach (medial-to-lateral) and infiltrate 1-3 ml both lateral and medial to the median nerve8,10
Landmark technique
- Inject site 1-2 cm proximal to the proximal wrist crease with the needle vertical just radial to the palmaris longus tendon or the midline (ulnar side of flexor carpi radialis) if the tendon is absent (palmaris longus is absent in up to 14% of the population)
- Advance the needle until there is no resistance
- Aspirate the needle to ensure that it is not in a blood vessel
- If parasthesia is felt do not inject (this indicates that the needle lies within the nerve and will cause damage)
- Inject 2-3 ml of lignocaine slowly (should be easy to inject if in the right place)
- Allow up to 10 minutes for the block to become effective. If the area still has some sensation, a repeat injection can improve the effect (after another 5-10 minutes).
Documentation
- All medication (including local anaesthetic) must be prescribed on the WA paediatric Hospital Medication Chart (pHMC).
- A procedure note should be entered into the patient medical record.
- It is important to document:
- Pre-procedure pain score
- Side of median nerve block
- Use of ultrasound or anatomical landmarks
- Needle type and size
- Local anaesthetic agent and dose
- Immediate complications
- Post nerve block pain scores should be performed and documented in the age-appropriate Observation and Response Tool 30 minutes after the procedure.
Bibliography
- Fleisher GR, Ludwig S. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2010.
- Royal Children’s Hospital Melbourne :Lidocaine (lignocaine) | Paediatric Injectable Guidelines Online (health.wa.gov.au). Update 2024. Cited 8 April 2025. Available from: Lidocaine (lignocaine) | Paediatric Injectable Guidelines Online (health.wa.gov.au)
- Australian Injectable Drugs Handbook, 9th Edition, 2025.Lidocaine hydrochloride. Last updated 20 March 2025. Cited 8 April 2025. Available from: AIDH - LIDOCAINE HYDROCHLORIDE (health.wa.gov.au)
- Australian Medicines Handbook [Internet]. Adelaide: AMH; c2022. Lidocaine. Available from: Lidocaine (anaesthesia) - Australian Medicines Handbook (health.wa.gov.au)
- Lidocaine (local and regional anaesthetic) and (systemic): Drug information. in: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on 8 April 2025.)
- Lidocaine and epinephrine: Drug information. in: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on 8 April 2025.)
- Liu W, Liu J, Tan X, Wang S. Ultrasound-guided lower forearm median nerve block in open surgery for trigger thumb in 1- to 3-year-old children: A randomized trial. Pediatr Anesth. 2018;28:134-141. https://doi.org/10.1111/pan.13296
- Strakowski JA. Ultrasound-Guided Peripheral Nerve Procedures. Phys Med Rehabil Clin N Am 2016;27:687-715.
- Frenkel O, Liebmann O, Fischer JW. Ultrasound-Guided Forearm Nerve Blocks in Kids: a novel method for pain control in the treatment of hand injured pediatric patients in the emergency department. Pediatr Emerg Care. 2015;31:296-307.
- Liebmann O, Price D, Mills C, Gardner R, Wang R, Wilson S, Gray A. Feasibility of Forearm Ultrasonography-Guided Nerve Blocks of the Radial, Ulnar, and Median Nerves for Hand Procedures in the Emergency Department. Ann Emerg Med 2006;48(5):558-562.
| Endorsed by: |
CAHS Drugs and Therapeutics Committee |
Date: |
Dec 2025 |
This document can be made available in alternative formats on request for people with disability.